Highly advanced, more accessible digital surgical technology.
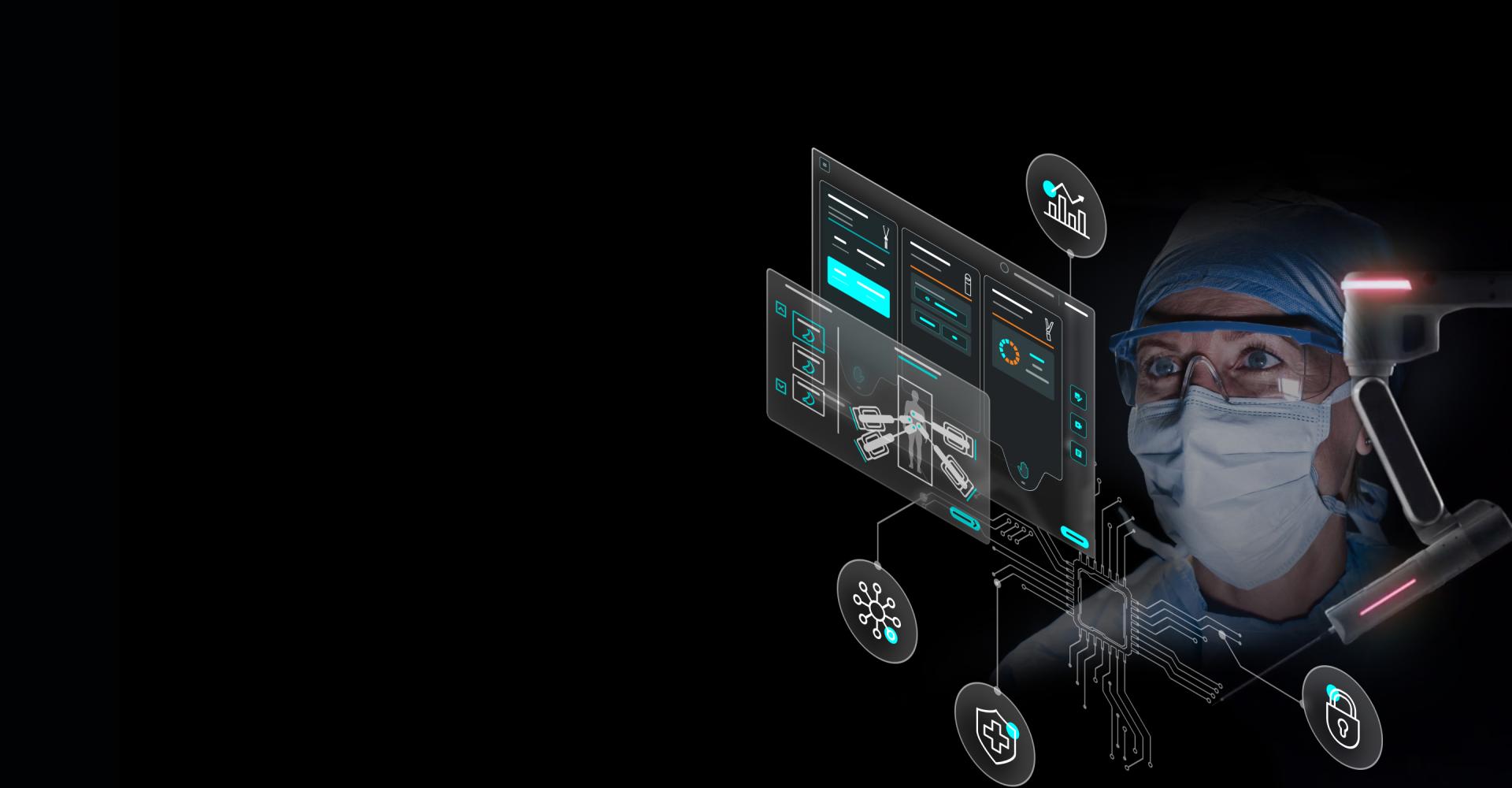
Actionable insights, delivered exactly when they're most valuable. Asensus Surgical is revolutionizing what’s possible so surgeons can perform safer, more predictable procedures.
Global Collaboration for Smarter Surgeries
Surgeons need real-time information to make informed decisions, navigate challenging anatomy, and reduce variability. That’s why our dedicated international team and physician partners around the world create digital and robotic technologies that go beyond hardware upgrades to enhance decision-making in the OR. By layering in advanced robotics and reusable instruments, we aim to provide the missing piece for the best standard of care, everywhere.
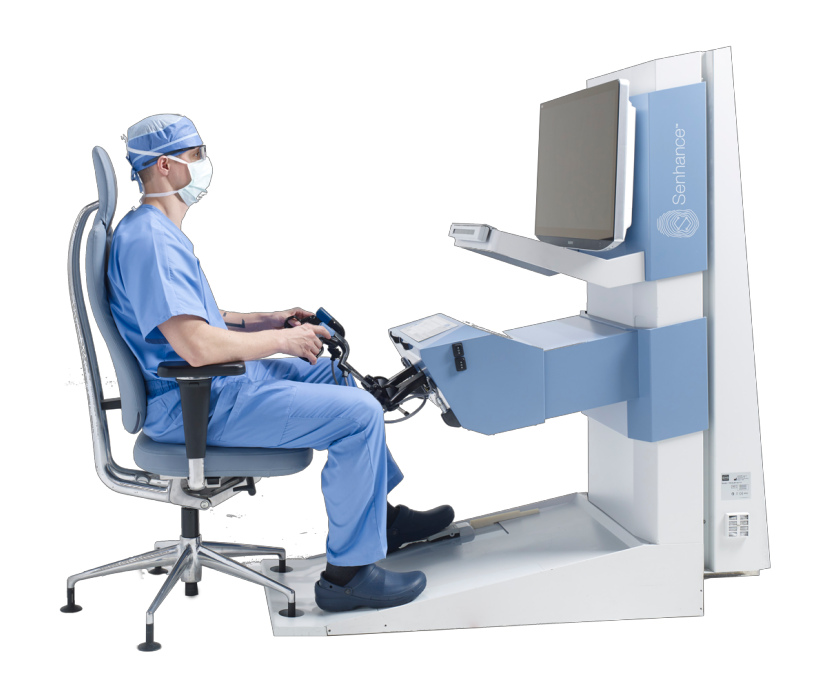
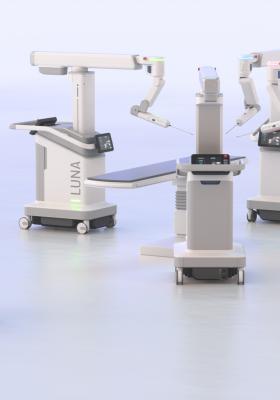
A Revolutionary Company, at the Forefront of Surgery
Groundbreaking robotic-assisted surgical technology, without the traditional barriers. The first intra-operative Augmented Intelligence system to be approved for use around the world. Asensus is disrupting differently to make the safest surgeries more accessible.